â… .DESCRIPTION
Aluminum Bitumen Butyl Tape is a cold applied self adhesive aluminum backed flashing in tape form.
â…¡.USES
Window and door openings (headers, sills,jambs, thresholds, nailing flanges)
Deck-to-wall intersections
Corner boards
Wall-to-wall tie-ins
Foundation sill plates
Sheathing panel seams
Under stucco finishes
Masonry walls
Roof detail areas
Gutters
Mobile home repair
Other building joints.
Exposed pipelines protection.
â…¢. COMPOSITION
High melting point polymer bitumen adhesive laminated to a 30-70 micron aluminium. The adhesive surface is protected by a release coated plastics film which is discarded during use.
â…£.Features
Easy application-Installation fast and easy-simply remove the release film and press onto the substrate.
Superior adhesion capabilities-Creates a strong bond to the substrate for long-lasting waterproofing
Protection.
Excellent sealing performance-The specially formulated rubberized asphalt adhesive seals around fasteners, allowing no water to penetrate and get to the substrate.
Highly conformable and flexible-Can accommodate settlement and shrinkage movement.
Long-lasting waterproofing protection-Both the aluminum surfaced polyethylene film and pure alu surface with the specially-formulated rubberized asphalt components create a water and moisture barrier that does not degrade from the effects of the environment.
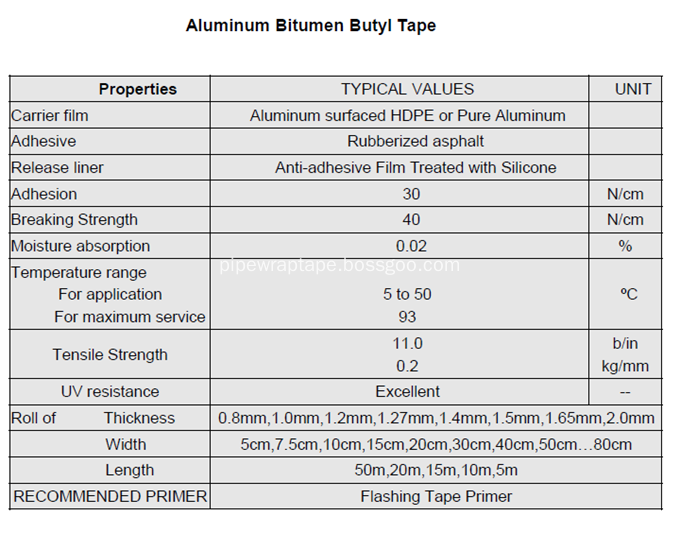
â…¤.Technical Datas:
Waterproofing Wrapping Tape, Waterproof Aluminium Bitumen Flashing Tape, Alu Flashing Tape Jining Xunda Pipe Coating Materials Co.,Ltd , http://www.pipe-wrap.com
Drexel University adds nanodiamonds to electrolytes to prevent the formation of dendritic deposits
Professor Bach and Dr. Yury Gogotsi of the University of Drexel School of Engineering led his team to publish an article entitled "Nano-diamond inhibition of lithium dendritic growth" in the journal Nature Communications. The study describes the shortening process technology of nano-diamond for electrochemical deposition, namely electroplating, which can cause short-circuit failure of lithium-ion batteries.
With the use of the battery and multiple charges, the electrochemical reaction that occurs causes the ions between the two electrodes in the cell to move; this repositioning of the ions produces a tendril-like buildup, just like accumulation in the cave. The same as the stalactites. The buildup in these batteries is called dendritic stone. Dendrites are the main cause of lithium battery failure. As the dendrites grow in the battery, they can penetrate the separator of the battery. The battery separator is a porous polymer film used to prevent the positive charge portion of the battery from coming into contact with the negative charge portion. When the separator is penetrated by dendrites, a short circuit occurs, and the highly flammable electrolyte inside the lithium battery burns, causing the battery to burn and malfunction.
In order to avoid the formation of dendrites and reduce the possibility of burning the battery, the current battery is designed to use a graphite electrode filled with lithium instead of a pure lithium electrode. This process of using graphite as a host for lithium prevents the formation of dendrites. However, the energy storage of lithium after doping graphite is about 10 times lower than that of pure lithium. Dr. Gogotsi's study used a pure lithium electrode to prepare a lithium battery, which not only eliminated the formation of dendrites, but also greatly improved the energy storage performance of the battery.
Dr. Gogotsi said: Battery safety is an important issue in our research. The mini disposable battery used in the electronic watch uses a lithium positive electrode, which is charged only once during the preparation process. However, if it is repeatedly charged, dendritic deposits will grow. Although lithium batteries have a certain safety cycle, repeated charging will eventually lead to battery short circuit. The purpose of our research is to make the lithium positive electrode more stable and to make the lithium plating more uniform, so that no dendritic deposits will grow.
The researchers first added nanodiamonds to the electrolyte solution, and during the deposition process, the nanodiamonds naturally detached together and formed a smooth surface. This property plays an important role in eliminating the formation of dendrites. Since lithium ions can easily adhere to nanodiamonds, the researchers used electroplating on the electrodes to electroplate the nanodiamonds. The electrolyte solution mixed with diamond significantly reduced the formation of dendrites in 100 charge cycles.
Gogotsi research found that this electrolyte with "additive" can produce a safer lithium battery with higher energy density and stable charge-discharge cycle for up to 200 hours, which is used in certain industries and military applications. The equipment is more than enough.

Abstract Drexel University adds nano-diamond to electrolyte solution to prevent the formation of dendritic deposits. Lithium-ion batteries are widely used in smart phones and notebook computers. They are the longest-life battery types in commercial batteries. However, in recent years, lithium batteries have frequently exploded and short-circuited batteries such as spontaneous combustion...
Lithium-ion batteries are widely used in smart phones and notebook computers, and are the longest-life battery types in commercial batteries. However, in recent years, lithium batteries have frequently caused various problems caused by short-circuiting of batteries such as malfunctions and spontaneous combustion. In response to this shortcoming of lithium batteries, Drexel University has recently developed a new technology to convert the key components of most batteries, electrolyte liquid, to avoid electrochemical reactions that cause battery safety accidents. happened.